And while the FDA has authorized the Pfizer Moderna and Johnson Johnson vaccines and is expected to also approve each one in turn for many people the. But now that the Moderna and Pfizer vaccines have been distributed by the millions the drug companies have even more evidence to present to.
Hahn said the authorization was given following an.

Are the vaccines fda approved. May 7 2021 1004 PM Ohio Gov. The FDA authorized the Pfizer-BioNTech vaccine for emergency use on Dec. WHAT WE FOUND According to the FDA An Emergency Use Authorization EUA is a mechanism to facilitate the availability and use of medical countermeasures including vaccines during public health.
Text in the photo reads FDA will not authorize or approve any COVID-19 vaccine The FDA however has already authorized three COVID-19 vaccines for emergency use. PFE and its partner BioNTech NASDAQ. Mike DeWine during a.
Approval means the FDA has officially decided that a product is safe and effective for its. BNTX recently announced that they plan to soon file for full US. Vaccines must be FDA approved for clinical testing in humans Vaccines that appear promising in pre-clinical trials where the vaccine is tested on tissue samples and in animal models have to be approved for a clinical trial before they can be tested on humans.
May 10 2021 -- Pfizer and its European partner BioNTech are seeking the FDAs full approval for their COVID-19 vaccine which is already being widely administered in. Food and Drug Administration FDA approval for their COVID-19 vaccine. A recurring sentiment among some in the vaccine-hesitant community is a desire to wait to receive any of the three available COVID-19 vaccines until they have been officially approved by the Food and Drug Administration.
Emergency use authorization is what its name suggests a medical product such as a vaccine that gets special FDA authorization to be used during an emergency. Currently no coronavirus vaccine is fully approved by the FDA but three were given emergency use authorization by the agency Published April 14 2021. However both Moderna and Johnson Johnson have indicated that.
Do the COVID-19 vaccines work. Pfizer asks US. When the health emergency is over.
Pfizer BioNTech Moderna and Johnson Johnson. The FDA amended the emergency use authorization of the Johnson Johnson Janssen COVID-19 vaccine to include information about a very rare and serious type of blood clot in people. Emergency use authorization is what its name suggests -- a medical product such as a vaccine that gets special FDA authorization to be used during an emergency.
All three FDA-authorized vaccines are effective in preventing COVID-19 and related serious outcomes including hospitalization and deaths. If the other COVID vaccines are still only being administered under emergency-use authorization they wont be able to remain on the market. 11 according to an agency news release.
CLEVELAND So far only one vaccine maker has submitted for full approval from the Food and Drug Administration FDA but the other two. When the health emergency is over. As vaccines approach full FDA approval could employers require workers to get the shot.
91 Zeilen April 23 2021. If Pfizers vaccine receives full approval from the FDA this will allow it to remain on the market even as the COVID pandemic comes to an end and is no longer considered an emergency. Approval No COVID-19 vaccines have been approved.
The approval would cover people ages 16 and up. FDA for full approval of COVID-19 vaccine - National Globalnewsca If approved the vaccine will be the first fully approved COVID-19. Although the companies expect the FDA any day to allow them to begin providing their vaccine to children 12-15 they will only ask for full approval.
No none of the COVID-19 vaccines have received FDA approval yet however Pfizer Moderna and Johnson Johnson were all granted emergency use authorization EUA. An FDA approval for a vaccine means the agency has decided that its benefits outweigh the known risks following a review of the manufacturers testing results. The Pfizer COVID-19 vaccine is the only one that has emergency approval for 12-year-olds and older -- the FDA approved the Pfizer vaccine for.
 What S The Difference Between Fda Emergency Use Authorization And Fda Approval Unc Health Covid 19 Vaccine Hub
What S The Difference Between Fda Emergency Use Authorization And Fda Approval Unc Health Covid 19 Vaccine Hub
 Are Covid Vaccines Fda Approved Here S How The Process Works Nbc Chicago
Are Covid Vaccines Fda Approved Here S How The Process Works Nbc Chicago
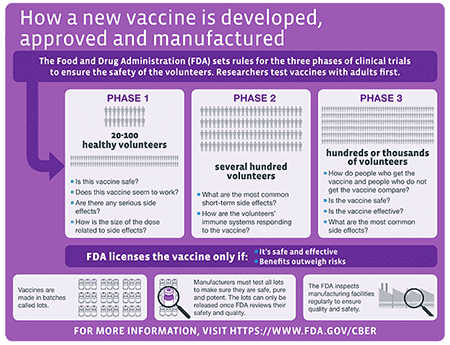 Ensuring The Safety Of Vaccines In The United States Cdc
Ensuring The Safety Of Vaccines In The United States Cdc
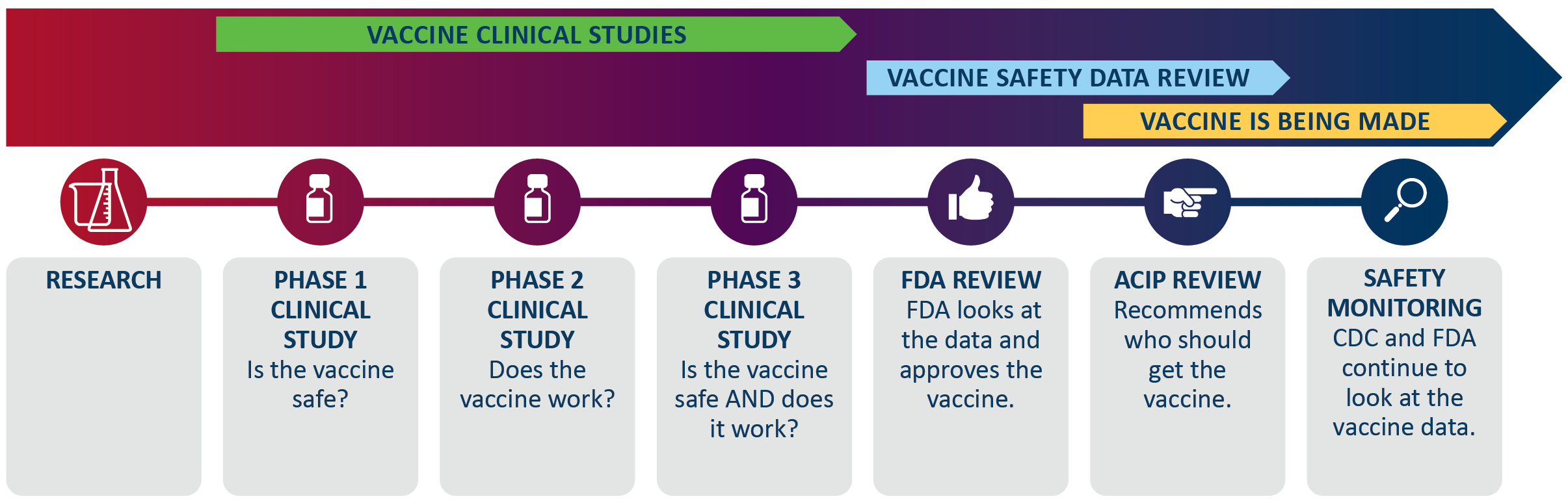 How Covid 19 Vaccines Are Made Immigrant Law Center Of Minnesota
How Covid 19 Vaccines Are Made Immigrant Law Center Of Minnesota
 F D A Clears Pfizer Vaccine And Millions Of Doses Will Be Shipped Right Away The New York Times
F D A Clears Pfizer Vaccine And Millions Of Doses Will Be Shipped Right Away The New York Times
 How The Fda Authorized Moderna Covid 19 Vaccine Compares To Pfizer S Science News
How The Fda Authorized Moderna Covid 19 Vaccine Compares To Pfizer S Science News
 Pfizer Seeks Us Fda Approval For Covid 19 Vaccine S Emergency Use Business And Economy News Al Jazeera
Pfizer Seeks Us Fda Approval For Covid 19 Vaccine S Emergency Use Business And Economy News Al Jazeera
 Learn More About Covid 19 Vaccines From The Fda Fda
Learn More About Covid 19 Vaccines From The Fda Fda
 Uk Approves Pfizer Biontech Covid 19 Vaccine Likely Signaling Fda Nod
Uk Approves Pfizer Biontech Covid 19 Vaccine Likely Signaling Fda Nod
Pfizer Biontech And Moderna Seek Full Fda Approval For Covid 19 Vaccines Biospace
 Medicare To Cover All Fda Approved Covid 19 Vaccines Medcity News
Medicare To Cover All Fda Approved Covid 19 Vaccines Medcity News
 Fda Approves Pfizer Biontech Coronavirus Vaccine For Emergency Use In Us Coronavirus The Guardian
Fda Approves Pfizer Biontech Coronavirus Vaccine For Emergency Use In Us Coronavirus The Guardian


Geen opmerkingen:
Een reactie posten
Opmerking: Alleen leden van deze blog kunnen een reactie posten.